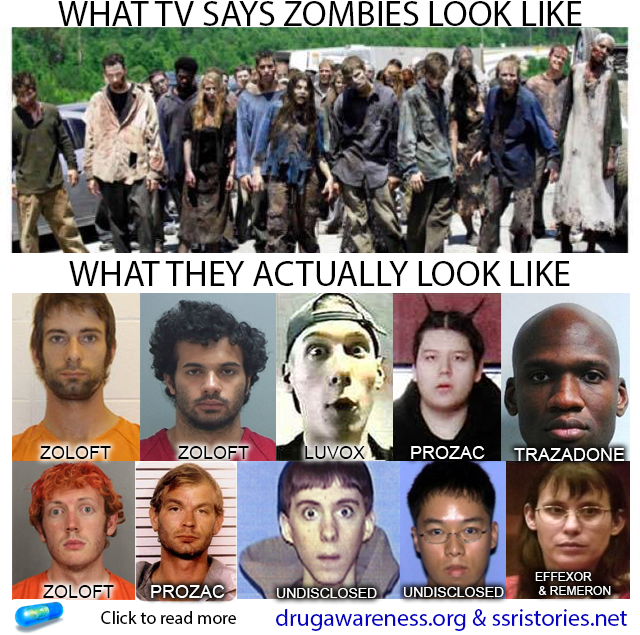
ANTIDEPRESSANTS AND THE ZOMBIE APOCALYPSE
If you click on the link and then go to each one of the pictures and click on them they will link to the articles discussing each case and the medications each person was on at the time of their crimes. Drugs which impair (inhibit) serotonin metabolism (SSRIs, SNRIs, Atypical Antipsychotics, etc.)




